Welcome to the Allergic Disease and Antiviral Responses Program at UT Southwestern Medical Center
Our goal is to understand the complex interaction between viral infections and allergic diseases
More Understanding, Better Healing
In a collaborative effort, Dr. David Farrar and Dr. Michelle Gill have
uncovered an important reciprocal negative regulatory pathway that may shed light on the devastating effects
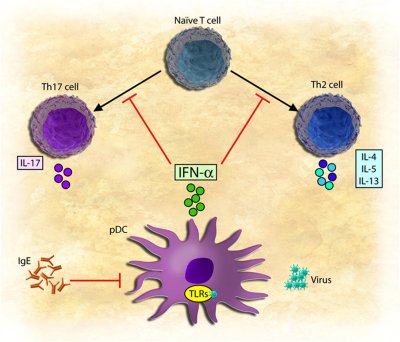
of viral infections on allergic diseases, such as allergic asthma. In response to viral infections, innate
cells of the immune system, called dendritic cells, secrete the antiviral cytokine, type I interferon
(IFN-α/β). This cytokine is critical in suppressing viral replication and spread to other cells. In turn,
dendritic cells prime populations of T cells to combat the virus by secreting inflammatory cytokines and by
providing help to B cells to produce antibody. This is the normal pathway that the immune system takes to
combat viral infections.
During allergic responses, T cells become primed to normally innocuous
substances such as pollens, dust, and pet dander, just to name a few. These aberrant T cells, called Th2
cells, drive the production of a highly inflammatory antibody type called IgE, which is the main mediator of
inflammation in allergic diseases. In our recent studies, we found that IFN-α/β, secreted during viral
infections, blocks the development of Th2 cells and can prevent them from secreting inflammatory cytokines.
Conversely, the product of the Th2 pathway, IgE, blocks the secretion of IFN-α/β from dendritic cells
responding to viral infections.
Thus, the very innate cytokine that blocks the development of allergic T cells
is suppressed by IgE-mediated allergic stimulation. Consequently, this pathway could reinforce priming of
allergic T cells through viral infections, which we propose is most relevant during the initial stages of
atopy induction as well as in cases of viral exacerbations of allergic asthma. These discoveries raise some
very important questions. First, can IFN-α/β destabilize and suppress the activity of allergen-specific Th2
cells? If so, then we propose that either direct treatment with IFN-α/β or an upstream inducer of
IFN-α/β
secretion could be used to treat allergic conditions. However, if IFN-α/β cannot reverse the Th2 phenotype
in T cells from atopic individuals, then understanding the molecular lesion in these cells would provide
clues to the initial priming events and genetic contributions that lead to the allergic state. Second, what
is the mechanism underlying the suppression of IFN-α/β by allergic stimulation, and can viral-induced
IFN-α/β responses from innate cells be restored in allergic individuals? The Allergic Disease and
Antiviral
Response Programs seeks to answer these important questions with the ultimate goal of uncovering new avenues
for therapeutic interventions for allergic diseases.